- References for developing this Code of Practice
- SDM arrangement for eHR sharing
- Healthcare providers in eHRSS
- Access to eHRSS by healthcare professionals
- Sharable clinical data in initial phase of eHRSS sharing
- Code of Conduct / Practices of healthcare professionals and relevant registration ordinances
- Code of Practice of healthcare orginisations in Hong Kong
- Policies, guidelines & procedures and other relevant information released by eHR office for participating in eHRSS
- Roles and responsibilities of user administrator in the Electronic Health Record Sharing System (eHRSS)
- Reference from the office of the Privacy Commissioner for Personal Data (PCPD)
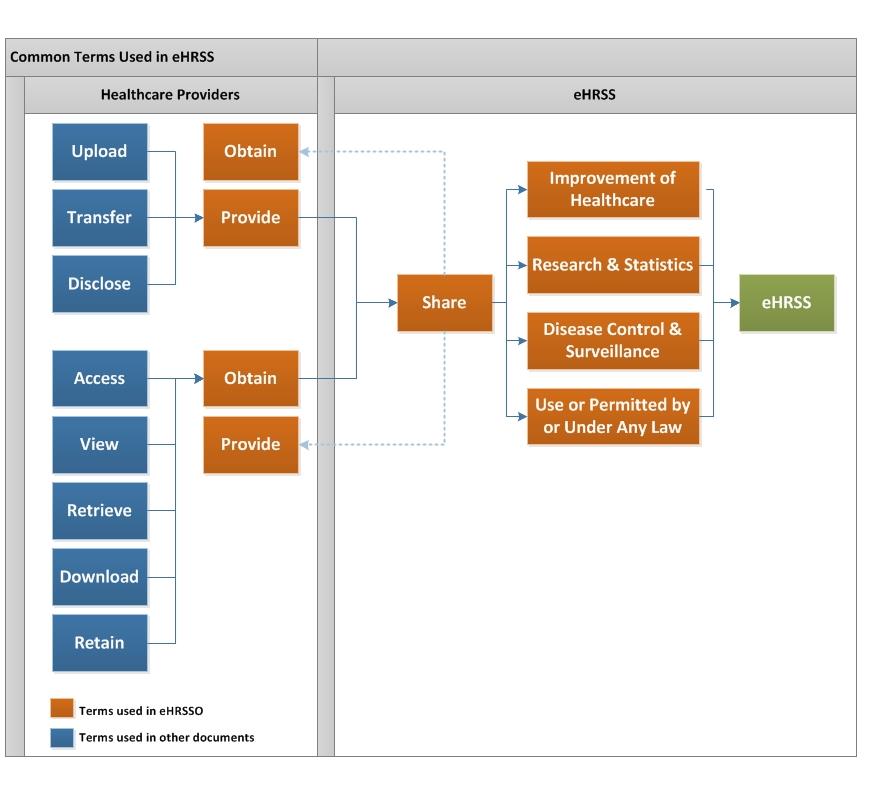
- Common terms about uses of eHR in eHRSS
1. References for developing this Code of Practice
- Advisory Guidelines For The Healthcare Sector. Singapore. 2014.
- Australian Medical Association. AMA Guide to Medical Practitioners on the use of the Personally Controlled Electronic Health Record System. Australia. 2012.
- Australian Government Minister for Health and Ageing. Concept of Operations: Relating to the Introduction of a Personally Controlled Electronic Health Record System. Australia. 2011.
- British Medical Association. Confidentiality and Disclosure of Health Information. Guidance from the BMA’s Medical Ethics Department. UK. 1999.
- British Medical Association and Royal College of General Practitioners. The Good Practice Guidelines for GP Electronic Patient Records. 2011
- British Psychological Society. Guidelines on the Use of Electronic Health Record. UK. 2011
- Canada Health Infoway Inc. White paper on Information Governance of the Interoperable Electronic Health Record (EHR). Canada. 2007
- Canada Health Infoway Inc. Electronic Health Record (EHR) Privacy and Security Requirements – Reviewed with Jurisdictions and Providers. Canada. 2005
- Code of conduct / practices of healthcare professionals and relevant registration ordinances in Hong Kong and Code of Practices issued by healthcare organisations in Hong Kong.
- Department of Health of Western Australia. Practice Code for the Use of Personal Health Information. Australia. 2009
- Department of Health. Contracted Service Providers Participating in the eHealth Record System: Frequently Asked Questions. Australia. 2013
- Department of Health. Participation Agreement. Personally Controlled Electronic Health Record System. Australia. 2013
- General Medical Council. Good Medical Practice. UK. 2006.
- General Medical Council. Confidentiality. UK. 2009.
- Hong Kong Medical Association & Independent Commission Against Corruption. Integrity in Practice. A Practical Guide for Medical Practitioners on Corruption Prevention.
- Information Commissioner’s Office. Data Sharing Code of Practice. UK. 2011
- Information and Privacy Commissioner. A Guide to Personal Health Information Protection Act. Ontario. 2004
- Medical Board of Australia. Good Medical Practice: A Code of Conduct for Doctors in Australia. Australia. 2009.
- National Health Service. The Care Record Guarantee. Our Guarantee for NHS Care Records in England. 2009
- National Health Service. Confidentiality. NHS Code of Practice. UK. 2003.
- National Health Service. Information Security Management - NHS Code of Practice. UK. 2007
- Office of Privacy Commissioner. On the Record. A Practical Guide to Health Information Privacy. New Zealand. 2011.
- Personal Data Protection Commission. A History and Theory of Informed Consent. New York: Oxford University Press. 1986
- Principles of Biomedical Ethics. New York: Oxford University Press 1994.
- Policies, guidelines & procedures and other relevant information released by the eHR Office for participating in eHRSS
- References from the Office of the Privacy Commissioner for Personal Data (PCPD)
2. Substitute decision maker (SDM) arrangement for electronic health record (eHR) sharing
Healthcare recipient under 16, or aged 16 or above and who is of any of the following, an eligible SDM can act on the person’s behalf at the person’s best interest on the following relevant occasions: giving joining consent and sharing consent, renewal or revocation of a sharing consent and request for withdrawal of participation in eHRSS.
| Persons incapable of giving consent | Persons who may act as SDM |
|---|---|
| (a) A minor (below 16 years old) |
|
| (b) Healthcare recipient who is 16 years old or above and is incapable of giving the person's own consent2 |
|
1 Appointed under Guardianship of Minors Ordinance (Cap 13) or appointed by court 2 Mentally incapacitated as defined by Mental Health Ordinance (Cap 136) s2(1);
Incapable of managing his or her own affairs;
Incapable of giving joining consent at relevant time;
Incapable of giving sharing consent at relevant time 3 Appointed under Mental Health Ordinance (Cap 136) 4 Under MHO s44A(1)(i), 44B(2A) or 59T(1) or 44B(2B) or 59T(2)
3. Healthcare providers in eHRSS
Healthcare provider that provides healthcare at one service location in Hong Kong may apply to the Commissioner to be registered as a healthcare provider for the System for the location.
A person provides healthcare at one service location if the person –
- is licensed under the Private Healthcare Facilities Ordinance (Cap. 633) in respect of one private healthcare facility; (Replaced 34 of 2018 s. 196 and E.R. 5 of 2018)
- is registered under section 5(2) of the Medical Clinics Ordinance (Cap 343) in respect of one clinic;
- carries on the business of dentistry under section 12 of the Dentists Registration Ordinance (Cap 156) at one place;
- holds a certificate of exemption issued under section 7(2), or a licence issued under section 8(2)(a), of the Residential Care Homes (Elderly Persons) Ordinance (Cap 459) in respect of one residential home and engages a healthcare professional;
- holds a licence issued under section 7(2)(a), or a certificate of exemption issued under section 11(2)(a), of the Residential Care Homes (Persons with Disabilities) Ordinance (Cap 613) in respect of one residential home for persons with disabilities and engages a healthcare professional; or
- is a specified entity that engages a healthcare professional to perform healthcare at one premises
The Commissioner may register a Government department as a healthcare provider for the System if the Commissioner is satisfied that the department provides a healthcare professional to perform healthcare for any healthcare recipient.
4. Access to eHRSS by healthcare professionals5
Access to eHRSS would be allowed for the following healthcare professionals:
- Registered medical practitioner (Cap 161);
- Registered nurse or enrolled nurse (Cap 164);
- Registered midwife (Cap 162);
- Registered dentist (Cap 156);
- Registered pharmacist (Cap 138);
- Registered medical laboratory technologist (Cap 359A);
- Registered radiographer (Cap 359H);
- Enrolled dental hygienist (Cap 156B);
- Registered chiropractor (Cap 428);
- Registered occupational therapist (Cap 359B);
- Registered Part I optometrist (Cap 359F);
- Registered physiotherapist (Cap 359J); and
- Registered and listed Chinese medicine practitioner (Cap 549).
Sharing by different healthcare professionals at different phases will be reviewed from time to time and announced by the eHR Office.
5 eHRSSO Cap 625, Schedule
5. Sharable clinical data in initial phase of eHRSS sharing
This section is extracted from the eHR Content Standards Guidebook.
Healthcare professionals should be aware that the eHRC will review, decide and update the sharable scope from time to time.
| eHR content | Definition |
|---|---|
| 1. Personal Identification and Demographic Data |
All information that is required to accurately and uniquely identify a person, including:
|
| 2. Encounters / Appointments |
A list of booked appointments and attended healthcare encounters (contact between a person and the healthcare practitioner who will assess, evaluate and treat a person). An episode is composed of one or more encounter(s). |
| 3. Healthcare Referrals |
Information that is required when a healthcare practitioner transfers all or a portion of a person’s care to another healthcare professional. |
| 4. Clinical Note / Summary |
The clinical note / summary contains information that summarise the following :
Remarks: The sharing of obstetric records by Healthcare Providers is available since March 2020. |
| 5. Allergies and Adverse Drug Reactions |
Information on the type of biological, physical or chemical agents that would result in / is proven to give rise to adverse health effects. Details of the adverse reactions, if occurred, should also be included. |
| 6. Diagnosis |
All active and inactive significant health and social problems. A problem can be a diagnosis, pathophysiological state, significant abnormal physical sign and examination finding, social problem, risk factor, allergy, reaction to drugs or foods, or health alert. |
| 7. Procedures |
Any significant procedures that are done for diagnosis, exploratory or treatment purposes. |
| 8. Birth Records |
The basic information about the eHR participant’s birth, e.g. place of birth, birth weight, maturity. Part of the information relating to birth would fall under the other sharable scope, e.g. diagnosis, procedure, assessment. |
| 9. Medication |
This includes medication ordered and/or dispensed/administered during the healthcare process. |
| 10. Immunisation Records |
Vaccines administered to the person. |
| 11. Laboratory Reports |
Result of the laboratory tests which are subclassified according to the nature of the test, namely anatomical pathology, biochemistry, haematology, microbiology, virology, and other laboratory test. |
| 12. Other Investigation Reports |
Other diagnostic test results could be of diverse range as discrete data element or a full report of the diagnostic test. Images, e.g. clinical photos, tracing, could also be included. |
| 13. Radiology Reports |
Radiology results would include radiology report and images. They are sub-classified according to modality, e.g. plain x-ray, fluoroscopy, ultrasound, computed tomography, magnetic resonance imaging, nuclear medicine, angiography and vascular interventional radiography, non-vascular interventional radiography, positive emission tomography and others. |
| 14. Medical Certificate |
A medical certificate is a formal statement about the health status or situation related to an individual. |
6. Code of Conduct / Practices of healthcare professionals and relevant registration ordinances
| Registered healthcare professionals | Documents | Issuing authority | Cap | Remarks |
|---|---|---|---|---|
| 1. Medical Practitioner | Code of Professionals Conduct for the Guidance of Registered Medical Practitioners English version Chinese version |
Medical Council of Hong Kong | Cap 161 | Revised in Jan 2016 |
| COP for Doctors (Private Hospital Association) English version |
Private Hospital Association | Revised in May 2020 | ||
| 2. Nurses (registered or enrolled) | Code of Ethics and Professional Conduct for Nurses in Hong Kong Bilingual version |
The Nursing Council of Hong Kong | Cap 164 | Revised in Jan 2015 |
| 3. Dentist | Code of Professional Discipline for the Guidance of Dental Practitioners in Hong Kong English version Chinese version |
The Dental Council of Hong Kong | Cap 156 | Revised in Dec 2019 |
| 4. Dental Hygienists | No information found in the website of the Dental Council of Hong Kong and there is no COP the Dental Hygienists | Cap 156B | ||
| 5. Chiropractors | Code of Practice for Registered Chiropractors English version Chinese version |
Chiropractors Council of Hong Kong | Cap 428 | Revised in Jan 2017 |
| 6. Midwife | Code of Professional Conduct and Practice for Midwives in Hong Kong English version Chinese version |
Midwives Council of Hong Kong | Cap 162 | Revised in Mar 2016 |
| 7. Pharmacist | Code of Professional Conduct for the Guidance of Registered Pharmacists in Hong Kong Bilingual version |
Pharmacy and Poisons Board of Hong Kong | Cap 138 | 2017 Version |
| 8. Medical Laboratory Technologist | Code of Practice for Registered Medical Laboratory Technologists English version Chinese version |
Medical Laboratory Technologists Board of Hong Kong | Cap 359A | Revised in April 2012 |
| 9. Occupational Therapist | Code of Practice for Registered Occupational Therapists English version Chinese version |
The Occupational Therapists Board of Hong Kong | Cap 359B | Revised in July 2017 |
| 10. Optometrist | Code of Practice for Registered Optometrists English version Chinese version |
Optometrists Board of Hong Kong | Cap 359F | Revised in Apr 2022 |
| 11. Radiographer | Code of Practice for Registered Radiographers English version Chinese version |
The Radiographers Board of Hong Kong | Cap 359H | 1998 Version |
| 12. Physiotherapist | Code of Practice for Registered Physiotherapists English version Chinese version |
The Physiotherapists Board of Hong Kong | Cap 359J | Revised in Jan 2014 |
13. Chinese Medicine Practitioner
|
Code of Professional Conduct for Registered Chinese Medicine Practitioners in Hong Kong English version Chinese version |
Chinese Medicine Council of Hong Kong | Cap 549 | Revised in July 2015 |
| Code of Conduct for Listed Chinese Medicine Practitioners English version Chinese version |
Revised in Jan 2018 |
7. Code of Practice of healthcare orginisations in Hong Kong
| Documents | Issuing Authority | Cap | Remarks | |
|---|---|---|---|---|
| 1. Clinics registered under the Medical Clinics Ordinance under Medical Clinics Ordinance (Cap 343) | Code of Practice for Clinics Registered Under The Medical Clinics Ordinance (Cap 343) English version Chinese version |
Department of Health | Cap 343 | 2010 Version |
8. Policies, guidelines & procedures and other relevant information released by eHR office for participating in eHRSS*
General Policies and Guidelines
- Participant Information Notice
- Conditions of Registration of Healthcare Providers (Private Hospitals) in eHRSS/Conditions of Registration of Healthcare Providers (other than Private Hospitals) in eHRSS
- eHRSS Privacy Policy Statement
- eHRSS Personal Information Collection Statements
- Important Note For Healthcare Providers Who Perform Healthcare Recipient Registration And / Or Obtaining Joining And Sharing Consent In Electronic Health Record Sharing System
- Training materials for the operation of registration centres
- Guidelines on Management of Healthcare Recipient Index
- Guidelines on Management of Healthcare Recipient (HCR) Data by Healthcare Providers
- eHRSS Data Retention Policy
- Policy and Guidelines for Handling Data Access Request (DAR) and Data Correction Request (DCR) in eHRSS
- Guidelines and procedures for using Hong Kong Identity (HKID) Card for eHR
- Frequently Asked Questions for eHRSS
eHR Data Standards
- eHR Content Standards Guidebook.
- Editorial Guide on Hong Kong Clinical Terminology Table – Overview
- Guide on Implementation & Maintenance of the Hong Kong Clinical Terminology Table
eHR Security and System Connection Guidelines
- IT Security Policies for eHRSS
- Security Assessment Checklist for Participating in the eHRSS
- Data Interoperability Guide
- Communication Protocol (Data Interface) Specification
- Encapsulated Linkage Security Application ("ELSA") Installation Guide
- eHR Adaptor Interface Specification
- Process Report and Exceptional Reporting Requirement
*The policies, guidelines & procedures and other relevant information released maybe updated from time to time. Please visit www.ehealth.gov.hk or contact the eHR Office for the latest information.
9. Roles and responsibilities of user administrator in the Electronic Health Record Sharing System (eHRSS)
- To manage user accounts and related administrative matters for healthcare providers to facilitate their participation and use of eHRSS. Such activities may include creation of user accounts with appropriate functions; proper assignment of access rights; maintenance of updated user list, user profile and professional registration information; and timely closure of user account(s) when necessary, etc.;
- To act as liaison person to communicate and cooperate with the Commissioner for the Electronic Health Record (eHRC) on matters relating to the use of eHRSS including:
- To collect feedback from users and to disseminate updates of eHRSS operational information to users (including provision of training on proper use of the eHRSS as and when required)
- To handle enquiries or complaints;
- To handle Data Correction Requests in accordance with Personal Data (Privacy) Ordinance (Cap. 486);
- To report to eHRC any suspected or confirmed data, privacy or security incidents;
- To provide assistance and information to eHRC and other relevant parties/agencies for facilitating the investigation of data, privacy or security incidents; and
- To carry out regular audit, investigation and related matters.
- To perform other administrative duties for the healthcare providers as required by eHRC for the effective operation of eHRSS.
10. Reference from the office of the Privacy Commissioner for Personal Data (PCPD)6
- Office of the Privacy Commissioner for Personal Data
- Personal Data (Privacy) Ordinance (Cap. 486)
- Data Protection Principles
- Code of Practice and Guideines
- Guidance Note
- Information Leaflets
- Proper Handling of Data Access Request and Charging of Data Access Request Fee by Data Users
- Guidance on the Proper Handling of Data Correction Request by Data Users
- Code of Practice on the Identity Card Number and other Personal Identifiers - Compliance Guide for Data Users (July 2016)
6 The references from PCPD listed in this COP are not exhaustive. PCPD may issue more Code of Practice or Guideline Note or other material or update any existing documents from time to time. Readers of this COP are advised to visit the internet website of the Office of the Privacy Commissioner for Personal Data for the latest information.
11. Common terms about uses of eHR in eHRSS